Surface modification of metallic components utilized within the life sciences sector is a critical process. This encompasses a range of techniques designed to enhance the properties of metallic parts, improving their resistance to corrosion, wear, and biological interaction. Examples include electropolishing of stainless steel bioreactors to create a smooth, easily cleanable surface and applying coatings to instruments to improve biocompatibility.
The practice holds significant importance due to the stringent requirements of the life sciences field. Enhanced corrosion resistance prevents contamination of sensitive biological samples. Improved wear resistance extends the lifespan of expensive equipment, reducing operational costs. Modified surfaces can also minimize protein adsorption and bacterial adhesion, which is vital for maintaining the integrity of experiments and ensuring patient safety. Historically, focus has been on preventing corrosion; however, contemporary applications demand tailored surface characteristics for advanced biotechnological processes.
The subsequent sections will delve into specific techniques employed in this domain, explore relevant regulatory considerations, and examine emerging trends shaping the future of surface engineering in the pursuit of advancements for biological research and pharmaceutical production. The choice of methods relies on the intended application and the metallic substrate in question.
Key Considerations for Surface Modification in Life Sciences
The following points offer essential guidance when considering surface engineering for metallic components within biotechnological applications.
Tip 1: Material Selection is Paramount: Choosing the appropriate metallic substrate is fundamental. Stainless steel grades 316 and 304 are commonly used due to corrosion resistance, but specific alloys may offer superior performance in particular environments.
Tip 2: Understand Regulatory Requirements: Surface treatments intended for contact with biological materials or pharmaceuticals must adhere to stringent regulatory standards, such as those established by the FDA and ISO. Compliance documentation is essential.
Tip 3: Consider the Intended Application: The final application dictates the required surface properties. For example, bioreactors require smooth, easily cleanable surfaces to prevent contamination, while surgical instruments may necessitate enhanced hardness and wear resistance.
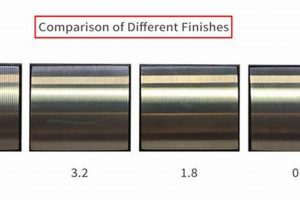
Tip 4: Evaluate Surface Roughness: Surface roughness significantly impacts protein adhesion and bacterial colonization. Achieving a controlled surface finish, often through electropolishing or precision grinding, is critical.
Tip 5: Explore Coating Options: A range of coatings, including biocompatible polymers and ceramics, can impart specific functionalities. Consider coatings that minimize protein adsorption, enhance corrosion resistance, or provide antimicrobial properties.
Tip 6: Validate Cleaning and Sterilization Procedures: Effective cleaning and sterilization protocols are essential to maintain the integrity of modified surfaces. Ensure that chosen cleaning agents and sterilization methods are compatible with the selected surface treatment.
Tip 7: Document All Processes: Meticulous documentation of all surface treatment processes, including materials used, process parameters, and quality control measures, is crucial for traceability and compliance.
Adhering to these guidelines promotes the successful integration of surface-engineered metallic components, ensuring optimal performance and regulatory adherence within the life sciences sector.
The subsequent sections will explore case studies and examples of these techniques in practice.
1. Corrosion Resistance
Corrosion resistance is paramount in the biotechnological sector, where metallic components are frequently exposed to aggressive chemical environments, including strong acids, bases, and sterilizing agents. Failure to adequately address corrosion can result in equipment degradation, process contamination, and potential safety hazards. Surface treatment serves as a critical line of defense against such degradation.
- Material Selection and Passivation
The inherent corrosion resistance of the base metal is the first line of defense. Stainless steel alloys, such as 316L, are frequently selected for their chromium content, which forms a passive oxide layer that inhibits corrosion. Post-fabrication, passivation treatments, often involving nitric acid, further enhance this layer, maximizing resistance to localized corrosion forms like pitting and crevice corrosion.
- Electropolishing and Surface Smoothing
Surface irregularities, such as scratches and machining marks, can act as initiation sites for corrosion. Electropolishing, an electrochemical process, selectively removes surface metal, resulting in a smoother, more uniform surface. This reduction in surface roughness minimizes the potential for corrosive agents to accumulate and initiate attack.
- Protective Coatings
In situations where the base metal’s inherent corrosion resistance is insufficient, protective coatings can be applied. These coatings, which can include polymers, ceramics, or even other metals, create a barrier between the metallic substrate and the corrosive environment. Selecting an appropriate coating requires careful consideration of the specific chemicals and temperatures encountered during operation.
- Controlled Environments and Process Monitoring
Surface treatment is not the sole determinant of corrosion resistance. Maintaining controlled environments, such as minimizing exposure to humidity and chlorides, and implementing regular process monitoring can also play a significant role. Periodic inspections and electrochemical testing can help detect early signs of corrosion and allow for timely intervention, preventing catastrophic equipment failure.
The interplay between these facets underscores the importance of a holistic approach to corrosion resistance in the biotechnology sector. Effective surface treatment, combined with appropriate material selection, environmental control, and monitoring, ensures the longevity and reliability of critical equipment, safeguarding product quality and operational efficiency.
2. Surface Biocompatibility
Surface biocompatibility is a critical parameter in the life sciences and a direct consequence of surface engineering in the metal finishing process. The interaction between a metallic implant or instrument and biological tissues or fluids depends heavily on the characteristics of the metal’s surface. An incompatible surface can trigger adverse reactions, including inflammation, thrombosis, or implant rejection. Therefore, surface modification techniques are employed to create a biocompatible interface.
Techniques such as plasma spraying, chemical vapor deposition, and electrochemical treatments alter the surface chemistry and topography to promote cell adhesion, prevent protein adsorption, or reduce bacterial colonization. For example, titanium implants often undergo surface roughening to enhance osseointegration, the direct structural and functional connection between living bone and the implant surface. Conversely, surfaces in contact with blood, such as those in cardiovascular devices, require treatments that minimize platelet adhesion and activation, preventing the formation of blood clots. The choice of surface modification depends on the specific application and the desired biological response.
In conclusion, surface biocompatibility is an engineered property, achieved through precise control of the metal finishing process. Understanding the relationship between surface characteristics and biological interactions allows for the development of medical devices and instruments that are safe, effective, and durable. The ongoing refinement of surface engineering techniques remains a central focus in the advancement of biotechnological applications.
3. Cleanability
Maintaining sterile conditions is paramount within the biotechnology industry, necessitating meticulous cleaning and sterilization protocols for all equipment and surfaces. Surface treatments significantly impact the ease and effectiveness of cleaning, directly influencing process integrity and product purity. Smooth, defect-free surfaces are crucial for preventing the accumulation of contaminants and facilitating thorough cleaning.
- Surface Roughness and Contaminant Retention
Surface roughness directly correlates with the potential for contaminant retention. Microscopic crevices and imperfections provide havens for bacteria, proteins, and other organic matter, making effective cleaning more challenging. Surface treatments that reduce roughness, such as electropolishing, are essential for minimizing these retention sites. Examples include the electropolished interiors of bioreactors, where a smooth surface is critical for preventing microbial growth and ensuring batch-to-batch consistency.
- Material Compatibility with Cleaning Agents
The compatibility of the surface treatment with commonly used cleaning and sterilizing agents is another crucial consideration. Certain treatments may degrade or corrode upon exposure to harsh chemicals, compromising the surface’s integrity and potentially releasing contaminants. Surface treatments must be carefully selected to withstand repeated exposure to cleaning agents like sodium hydroxide, nitric acid, and peracetic acid, which are routinely used in biopharmaceutical manufacturing.
- Passivation Layer Integrity
For stainless steel components, the integrity of the passive chromium oxide layer is essential for maintaining corrosion resistance and cleanability. Harsh cleaning procedures or improper handling can damage this layer, rendering the surface more susceptible to corrosion and increasing the risk of contaminant retention. Surface treatments that enhance the stability and regeneration of the passive layer contribute to improved cleanability and long-term performance.
- Surface Energy and Fouling Resistance
Surface energy influences the adhesion of contaminants. Low surface energy materials tend to resist fouling, making them easier to clean. Applying coatings with low surface energy characteristics can improve the cleanability of metallic components. For instance, fluoropolymer coatings are sometimes used on components exposed to sticky or viscous materials, facilitating their removal during cleaning cycles.
The preceding facets highlight the integral role of surface treatments in achieving and maintaining cleanability in the biotechnology industry. The careful selection and application of appropriate surface modification techniques are indispensable for ensuring the integrity of bioprocesses and the quality of biopharmaceutical products. The ability to effectively clean and sterilize equipment is a cornerstone of safe and efficient operation within this sector.
4. Sterilization Compatibility
Surface modification profoundly affects the ability of metallic components to withstand sterilization procedures. Sterilization compatibility, therefore, becomes an intrinsic requirement when implementing surface treatments. The methods used to eliminate microorganisms, such as autoclaving, chemical sterilization, or irradiation, can cause degradation or alteration of surface finishes. The selection of an appropriate finish is contingent upon its resilience to the chosen sterilization technique. Failure to consider this leads to compromised surface integrity, potential leaching of materials into the process stream, and ultimately, a failure to achieve sterility.
For example, electropolishing, a common surface treatment for stainless steel equipment, enhances both corrosion resistance and cleanability, contributing to better sterilization outcomes. However, some coating materials, if improperly applied, might delaminate under the high-temperature steam of an autoclave. Similarly, certain anodized finishes on aluminum, while providing excellent corrosion resistance, might react adversely to strong chemical sterilants. The compatibility must also be considered in the light of repeated cycles: a finish that is initially compatible might degrade over time with repeated exposure. A real-world example exists in the case of porous coatings intended to promote osseointegration; these, if not properly designed, can trap contaminants and complicate sterilization.
In conclusion, surface treatments and sterilization procedures are inextricably linked. The selection of a finishing method must prioritize the ability of the finished surface to withstand the rigors of the sterilization process without compromising its integrity or introducing contaminants. Careful consideration of this interdependency is crucial for ensuring the safety and efficacy of processes within the biotechnology industry, thereby avoiding the risks associated with inadequate sterilization and potential product contamination.
5. Regulatory Adherence
Regulatory adherence forms a cornerstone within the biotechnology industry, particularly concerning processes such as surface engineering. Surface modifications of metallic components, directly influencing product purity and patient safety, are subject to rigorous regulatory scrutiny. Non-compliance can result in product recalls, legal penalties, and significant reputational damage. Therefore, a comprehensive understanding of applicable regulations is crucial.
- FDA Compliance and 21 CFR Part 11
The U.S. Food and Drug Administration (FDA) mandates strict control over manufacturing processes, including surface modification, under Current Good Manufacturing Practice (CGMP) regulations. Specifically, 21 CFR Part 11 governs electronic records and electronic signatures, requiring that all data related to surface treatments, such as process parameters and quality control results, be securely stored and auditable. Failure to maintain compliant records can lead to serious regulatory consequences. For instance, a medical device company that improperly documents electropolishing processes for implantable devices could face warning letters or even product recalls.
- ISO Standards and Certification
International Organization for Standardization (ISO) standards, such as ISO 13485 for medical device quality management systems, provide a framework for ensuring consistent product quality and regulatory compliance. Certification to ISO 13485 demonstrates a company’s commitment to adhering to internationally recognized best practices in manufacturing, including surface treatment processes. Many regulatory bodies, including the FDA, recognize ISO 13485 as evidence of compliance with applicable regulations. For example, achieving ISO 13485 certification for a facility that performs surface treatments on surgical instruments demonstrates a commitment to maintaining high-quality standards and regulatory compliance.
- REACH and RoHS Directives
The European Union’s Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and Restriction of Hazardous Substances (RoHS) directives restrict the use of certain hazardous substances in manufacturing processes and products. Surface treatments that involve the use of restricted substances, such as hexavalent chromium, must comply with these directives. Companies that fail to comply with REACH and RoHS risk fines, product recalls, and restricted access to the European market. An example is the replacement of chromate conversion coatings with alternative, RoHS-compliant finishes for aluminum components used in medical equipment.
- Material Traceability and Documentation
Maintaining complete traceability of materials used in surface treatment processes is essential for regulatory compliance. This includes tracking the source, composition, and processing history of all materials, from the raw metal substrate to the finishing chemicals used. Detailed documentation of all surface treatment steps, including process parameters, equipment calibration records, and operator training records, is also required. In the event of a product recall or regulatory audit, this documentation provides evidence of compliance with applicable regulations. For example, if a batch of surgical implants is found to have inadequate corrosion resistance, traceability records can be used to identify the source of the problem and prevent similar issues from occurring in the future.
The previously discussed facets highlight the critical importance of regulatory compliance in the field. Adherence to regulations is not merely a procedural requirement; it is a fundamental aspect of ensuring product safety, efficacy, and market access. Surface engineering companies must prioritize regulatory compliance in all aspects of their operations, from material selection and process design to quality control and documentation.
Frequently Asked Questions
The following questions address common concerns and misconceptions regarding surface treatments within the life sciences sector. The answers are designed to provide concise, informative guidance.
Question 1: What types of metal substrates are most commonly used?
Stainless steel alloys, particularly 316L and 304, are prevalent due to their inherent corrosion resistance and biocompatibility. Titanium and its alloys also see significant use, especially in implantable devices.
Question 2: How does electropolishing improve the performance of equipment?
Electropolishing reduces surface roughness, thereby minimizing contaminant adhesion and facilitating cleaning and sterilization. This improves corrosion resistance and prevents bacterial colonization.
Question 3: What regulatory standards govern surface treatments for medical devices?
Surface treatments intended for medical devices are subject to regulations from organizations such as the FDA (21 CFR Part 820) and ISO (ISO 13485), ensuring product quality and safety.
Question 4: Are all surface coatings biocompatible?
No. The biocompatibility of a surface coating must be carefully evaluated based on its chemical composition and intended application. Coatings designed for blood contact require different properties than those designed for bone integration.
Question 5: How does surface roughness impact sterilization effectiveness?
Increased surface roughness provides havens for microorganisms, making complete sterilization more difficult. Smooth surfaces achieved through techniques like electropolishing are more readily sterilized.
Question 6: What are the key considerations when selecting a surface treatment for a bioreactor?
The primary considerations for a bioreactor include resistance to corrosion from process fluids, ease of cleaning to prevent contamination, and compatibility with sterilization procedures, such as autoclaving or chemical sterilization.
In summary, optimal performance and regulatory compliance within the biotechnology industry hinge on careful consideration of material selection, surface treatment techniques, and adherence to relevant standards.
The next section will explore emerging trends shaping the future of surface modification in this field.
Conclusion
The preceding discussion has elucidated the critical role of surface engineering in the life sciences. The selection and application of appropriate metal finishing techniques are not merely cosmetic enhancements but rather integral components of ensuring product integrity, regulatory compliance, and ultimately, patient safety. The intricacies of corrosion resistance, biocompatibility, cleanability, sterilization compatibility, and regulatory adherence necessitate a comprehensive understanding of both surface science and the specific requirements of biotechnological applications.
Continued advancements in surface modification technologies promise to further refine and enhance the capabilities of metallic components within the biotechnology sector. A commitment to rigorous research, stringent quality control, and proactive engagement with evolving regulatory landscapes will be paramount in unlocking the full potential of these technologies and driving innovation in this vital field. Further exploration of specialized applications and emerging surface modification techniques is warranted to maintain competitiveness and advance the field.